One cycle of battery charging and discharging is called a cycle, and cycle life is an important indicator of battery life performance. The root cause of the factors affecting the cycle life of lithium-ion batteries is that the number of lithium ions involved in energy transfer is constantly decreasing. The total amount of lithium in the battery has not decreased, but the "activated" lithium ions are less, they are imprisoned in some places or the transmission channel is blocked, and they cannot freely participate in the charging and discharging process. Specifically include:
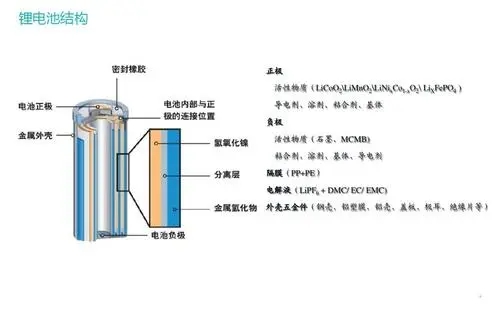
(1) Precipitation of metallic lithium: generally occurs on the surface of the negative electrode. When lithium ions migrate to the surface of the negative electrode, some of the lithium ions do not enter the negative electrode active material to form stable compounds, but instead obtain electrons and deposit on the negative electrode surface to become metal lithium, and It no longer participates in the subsequent cycle process, resulting in a decrease in capacity. For example, when overcharged or the negative electrode material is insufficient, the negative electrode cannot accommodate the lithium ions migrated from the positive electrode, resulting in the deposition of metallic lithium; during high-rate charging, due to the excessive number of lithium ions reaching the negative electrode in a short period of time, causing channel blockage and Precipitate.
(2) Decomposition of the positive electrode material: The lithium-containing metal oxide of the positive electrode material will continue to decompose during long-term use, producing some electrochemically inert substances and some flammable gases, destroying the capacity balance between the electrodes and causing the capacity to decrease. irreversible loss.
(3) SEI film on the electrode surface: carbon anode material, during the initial cycle, the electrolyte will form a solid electrolyte (SEI) film on the electrode surface, the formation of the SEI film will consume lithium ions, and the SEI film is not. Stable and constant, it will continue to rupture during the cycle, exposing the new negative electrode surface and then reacting with the electrolyte to form a new SEI film, which will continue to cause continuous loss of lithium ions and electrolytes, resulting in a decrease in battery capacity. In addition, the diffusion channels of lithium ions in the SEI film may be blocked, which will also cause a decrease in battery capacity.
(4) Loss of electrolyte: in the process of continuous circulation, the electrolyte will continue to decompose and volatilize, resulting in a decrease in the total amount of electrolyte, inability to fully infiltrate the positive and negative materials, and incomplete charge-discharge reaction, resulting in the actual use capacity. Decline. In addition, if the electrolyte contains a certain amount of water, the water will chemically react with LiFP6 to produce LiF and HF, which in turn destroys the SEI film, generating more LiF, causing LiF deposition, and continuously consuming active lithium ions. Decreased battery cycle life.
(5) Blockage or damage of the diaphragm: During the cycle of lithium-ion batteries, the gradual drying and failure of the diaphragm is also a cause of capacity decline. Due to the drying of the separator, the ohmic internal resistance of the battery increases, resulting in blockage of the charging and discharging channels, incomplete charging and discharging, and the battery capacity cannot be restored to the initial state, which greatly reduces the capacity and service life of the battery.
(6) The positive and negative materials fall off: the active materials of the positive and negative electrodes are fixed on the substrate by the binder. During long-term use, due to the failure of the binder and the mechanical vibration of the battery, the positive and negative electrodes are The active material of the battery is continuously falling off and entering the electrolyte solution, which leads to the continuous reduction of the active material that can participate in the electrochemical reaction, and the cycle life of the battery is continuously reduced. The long-term stability of the binder and the good mechanical properties of the battery will be able to delay the decline in the cycle life of the battery.
NO.3, Zhukeng Third Industrial Zone, Jinniu East Road, Pingshan District, Shenzhen
0755-89369630
400-822-5968
brand@sz-manst.com


